Why does ionization energy decrease from top to bottom. Electronegativity is the tendency of an atom to attract the electrons in a bond towards it while ionization energy is the energy a neutral atom needs to remove an electron from it.

Periodic Trends Chemwiki Electron Affinity Ionization Energy Chemistry Textbook
Electronegativity is an atoms tendency to attract an electron.

. Which has a higher first ionization energy. The atom experiences a jump in ionization energy because the electrons are closer to the protons in the nucleus. In contrast the ionization energy is the energy needed to remove an electron from an atom.
Phosphorous P or magnesium Mg. The first ionisation energy is the energy required to remove one mole of the most loosely held electrons from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1. Breaks in a trend may be due to.
Electronegativity and first ionization energy both increase going up the Periodic Table. Therefore electronegativity is directly related to that non-metallic properties of elements. Electronegativity increases as you move up the table whereas ionization energy decreases.
34 Atomic Trends Ionization Energy. Electronegativity decreases as you move up the table whereas ionization energy increases. If the difference in electronegativity is greater than 17 the character of the bond will be ionic.
The degree to which an atom attracts electrons in a chemical bond is described by electronegativity. An increase in ionization energy led to an increase in the ionization energy. Since having more tendency to attract an electron means that the atom has less tendency to give an electron more electronegative atoms require more energy to ionize.
If the radius is larger then those electrons on the outer edge of the atom arent being held in so close and are easier to lose - requiring a lower amount. The smaller the radius the higher the ionization energy. This depends on the number of protons and on the orbitals that the electron occupies.
Electronegativity is the attract electron while ionization energy is energy required to remove electron and hence if an atom has great electronegativity it cannot loss an electron easily. Whereas ionization energy increases from left to right in a row and. Which property has a trend.
How is the electronegativity trend related to the first ionization energy trend. If the difference in electronegativity is between 04 and 17 the character of the bond is polar covalent. How is the electronegativity trend related to the first ionization energy trend.
Electronegativity is practically the opposite of ionization energy - it increases as the atomic radius gets smaller since then its protons are closer to its electrons and thus are able to exert more attractive force on each other. Ionization always requires energy. This is because the electrons are being held in closer to the protons which have opposing charges and therefore hold on to them in an atom with a small radius.
As mentioned the electronegativity trend refers to the way electronegativity values trend across the periodic table of the elements. Atoms are the building blocks of all existing substances. Groups of the Table.
In general electronegativity decreases as you move down a group in the periodic table this correlates. View 34 Trendsdocx from CHEM 623162 at Round Rock H S. January 10 2012 Posted by Dunee.
When atoms are ionized they lose an electron and become positively charged. Actually the trend of electronegativity and first ionization energy is the same but just keep In mind there are several exceptions for example the first ionization energy of N is actually larger than O. Electronegativity and first ionization energy both decrease as you move up the periodic table.
An increase in ionization energy led to an increase in the ionization energy. It can be further extended to say that the electronegativity is inversely related to the metallic properties of elements. Thus the increase in.
The amount of energy required to separate one electron from its atom first ionization energy depends on how tightly held the electron is. Keelynnbarrier SHOW ANSWER The greater the electronegativity the greater the first ionization energy. The greater the electronegativity the greater the first ionization energy.
The ionization energy of the elements within a period generally increases from left to right. Answers The correct answer was given. Orbital symmetry based on the number of electrons What is electron affinity.
93 CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Non-metallic elements have strong tendency to gain electrons. When moving from left to right across the periodic table electronegativity increases with the exception being the noble gases. How IS The electronegativity trend relates to the first ionization energy trend.
The key difference between electronegativity and ionization energy is that electronegativity explains the attraction of electrons while ionization energy refers to the removal of electrons from an atom.

How Is Electronegativity Related To Ionization Energy And Electron Affinity Quora
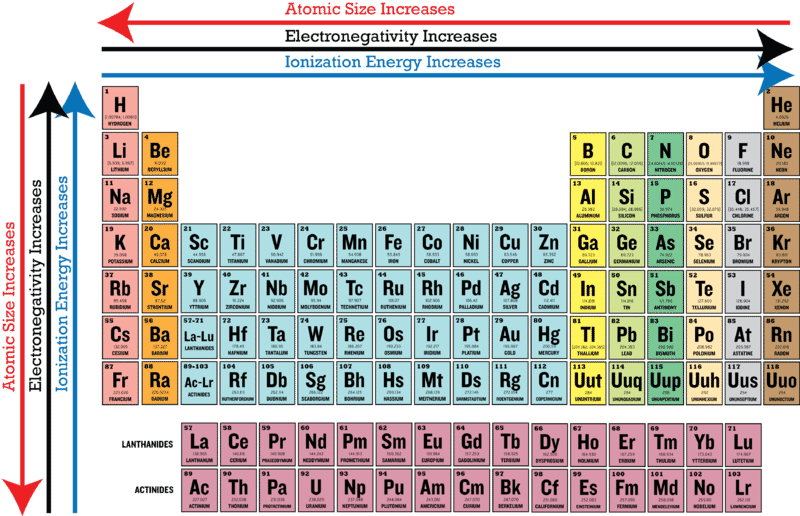
Periodic Trends In Electronegativity Ck 12 Foundation

Easy To Use Chart Of Periodic Table Trends Teaching Chemistry Chemistry Classroom Science Chemistry
0 Comments